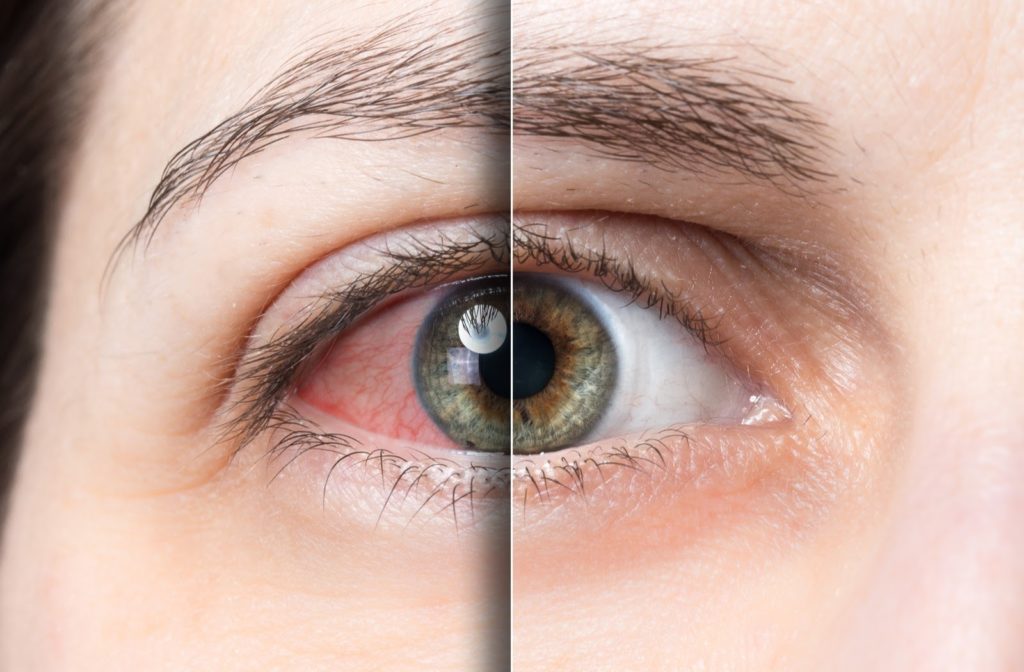
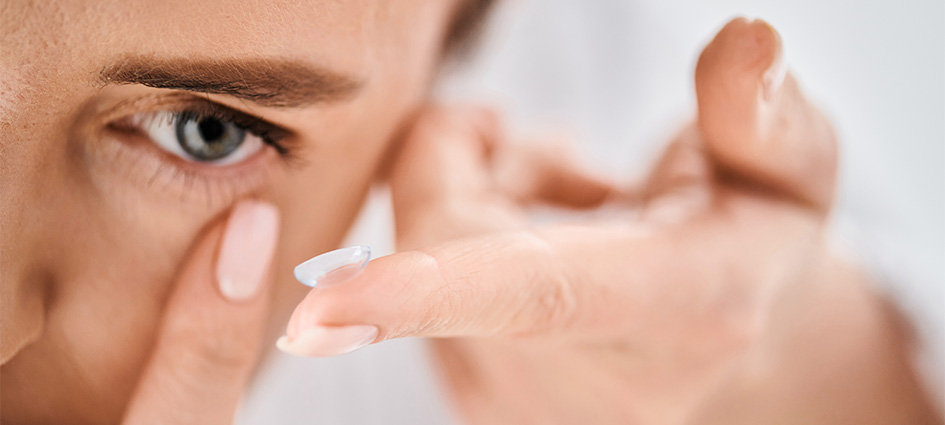
Childhood is a critical stage for overall development, and vision plays a crucial role in helping children learn, explore, and grow. But myopia is becoming increasingly common among young children worldwide. Fortunately, there are ways to minimize risk and keep myopia from worsening. A few practical strategies to slow myopia progression in children include: Increased […]
How to Slow Myopia Progression in Children